ETHANOL Extra Pure 99-99.5%
Price 1010 INR/ Milliliter
MOQ : 500 Milliliters
ETHANOL Extra Pure 99-99.5% Specification
- Ph Level
- Neutral (pH 7 at 100g/l, 20 C)
- Boiling point
- 78.3C
- Molecular Weight
- 46.07 g/mol
- Size
- 500 mL, 2.5 L, 5 L, 25 L
- Density
- 0.789 Gram per cubic centimeter(g/cm3)
- Physical State
- Liquid
- Melting Point
- -114.1C
- Molecular Formula
- C2H6O
- Storage Instructions
- Store in a cool, dry, well-ventilated area, away from ignition sources
- Shelf Life
- 36 Months
- Flash Point
- 13C (Closed cup)
- Packaging Type
- Bottle/Drum
- Usage
- Research, laboratory, and industrial use
- Purity
- Extra Pure
- CAS No
- 64-17-5
- Grade
- AR
- Type
- University Lab Chemicals
- Application
- Laboratory and analytical reagent, Industrial solvent, Pharmaceuticals
- Appearance
- Clear, colorless liquid
- Purity(%)
- 99.7-100 %
- HS Code
- 2207
- Solubility
- Miscible with water, ether, chloroform
- Odor
- Characteristic, alcohol-like
- Auto-ignition Temperature
- 363°C
- Hazard Class
- 3 (Flammable Liquid)
- Refractive Index
- 1.361 (at 20°C)
- Vapor Pressure
- 44 mmHg at 20°C
- UN Number
- 1170
- Evaporation Rate
- 2.93 (butyl acetate=1)
- Assay
- >=99.7%
ETHANOL Extra Pure 99-99.5% Trade Information
- Minimum Order Quantity
- 500 Milliliters
- FOB Port
- Nhava- sheva, india
- Payment Terms
- Cash Advance (CA), Cheque
- Supply Ability
- 50000 Milliliters Per Week
- Delivery Time
- 4-5 Days
- Sample Available
- Yes
- Sample Policy
- Sample costs shipping and taxes has to be paid by the buyer
- Packaging Details
- 25 Gm, 100 Gm, 250 Gm , 500 Gm ,1kg, 2.5kg, 5kg, 25kg , 50 kg, export worthy Packing
- Main Export Market(s)
- Western Europe, Middle East, Central America, South America, Asia, Eastern Europe, North America, Australia, Africa
- Main Domestic Market
- Dadra and Nagar Haveli, Andaman and Nicobar Islands, Pondicherry, Uttarakhand, Daman and Diu, Lakshadweep, Nagaland, , Sikkim, Chandigarh, Goa, Jharkhand, Manipur, South India, Arunachal Pradesh, Tamil Nadu, Punjab, Assam, Meghalaya, Mizoram, East India, Jammu and Kashmir, Maharashtra, Rajasthan, Madhya Pradesh, Andhra Pradesh, West Bengal, Haryana, Tripura, Kerala, Bihar, Odisha, Delhi, Gujarat, Karnataka, North India, Telangana, Central India, Uttar Pradesh, West India, Chhattisgarh, Himachal Pradesh, All India
- Certifications
- ISO 14001 : 2015 ISO 9001 : 2008 OHSAS 18001 : 2007 WHO GMP
About ETHANOL Extra Pure 99-99.5%
Ethanol (also called ethyl alcohol, grain alcohol, drinking alcohol, or simply alcohol) is a chemical compound, a simple alcohol with the chemical formula C2H6O. Ethanol is a volatile, flammable, colorless liquid with a slight characteristic odor. It is a psychoactive substance and is the principal active ingredient found in alcoholic drinks. It has medical applications as an antiseptic and disinfectant. The compound is widely used as a chemical solvent, either for scientific chemical testing or in synthesis of other organic compounds, and is a vital substance used across many different kinds of manufacturing industries.
Ethanol Technical Details:
Other names : Absolute alcohol, alcohol, cologne spirit, drinking alcohol, ethylic alcohol, EtOH, ethyl alcohol, ethyl hydrate, ethyl hydroxide, ethylol, grain alcohol, hydroxyethane, methylcarbinol
Chemical formula : C2H6O
Molar mass : 46.069 gmol1
Appearance : Colorless liquid
Density : 0.7893 g/cm3 (at 20 C)
Melting point : 114.14 0.03[2] C (173.45 0.05 F; 159.01 0.03 K)
Boiling point : 78.24 0.09[2] C (172.83 0.16 F; 351.39 0.09 K)
Solubility in water : miscible
log P : 0.18
Vapor pressure : 5.95 kPa (at 20 C)
Acidity (pKa) : 15.9 (H2O), 29.8 (DMSO)
Magnetic susceptibility () 33.60106 cm3/mol
Refractive index (nD) : 1.3611
Viscosity : 1.2 mPas (at 20 C), 1.074 mPas (at 25 C)
Dipole moment : 1.69 D
Exceptional Purity and Versatility
Ethanol Extra Pure 99-99.5% provides exceptional purity for laboratory, pharmaceutical, and industrial applications. Its remarkable solubility profile and reliability as an analytical reagent make it indispensable for researchers and technicians. This AR grade ethanol ensures consistent and accurate results whether used in research, as a solvent, or in pharmaceutical processes.
Safe Handling and Storage
Due to its high flammability (Flash Point: 13C, Hazard Class 3), Ethanol Extra Pure should be stored in a cool, dry, and well-ventilated area, far from ignition sources. Adhering to proper storage protocols preserves both user safety and product shelf life, which is rated at 36 months.
FAQ's of ETHANOL Extra Pure 99-99.5%:
Q: How should ETHANOL Extra Pure 99-99.5% be stored for maximum safety and longevity?
A: Store ETHANOL Extra Pure in a cool, dry, and well-ventilated space away from heat, spark, open flame, and incompatible materials. Proper storage ensures up to 36 months of shelf life and maintains product purity.Q: What are the common applications and benefits of using this grade of ethanol?
A: This extra pure ethanol is widely used as a laboratory reagent, industrial solvent, and in pharmaceutical processes. Its high purity ensures reliable results in analytical procedures, and its miscibility with water and organic solvents offers versatility for numerous research and production tasks.Q: When is it suitable to use ETHANOL Extra Pure instead of lower purity grades?
A: Use ETHANOL Extra Pure whenever analytical accuracy is essential, such as in research settings, pharmaceuticals, or where trace impurities could interfere with experimental or production outcomes. Its AR grade is especially valuable for critical laboratory and analytical protocols.Q: Where can ETHANOL Extra Pure 99-99.5% be applied in laboratory or industrial processes?
A: It is suitable for use in university laboratories, analytical chemistry, pharmaceuticals, and as an industrial solvent. Its purity level supports applications where moisture and contaminants must be minimized.Q: What packaging sizes are available for ETHANOL Extra Pure, and who can purchase it?
A: ETHANOL Extra Pure is supplied in bottles and drums, available in 500 mL, 2.5 L, 5 L, and 25 L volumes. It is suitable for dealers, exporters, manufacturers, retailers, suppliers, traders, and research institutions across India.Q: What precautions should be taken when handling ETHANOL Extra Pure in the lab?
A: Use appropriate personal protective equipment (PPE), work in well-ventilated areas, keep away from ignition sources, and follow all safety guidelines for handling flammable liquids. Proper handling prevents accidents and exposure.Q: How does ETHANOL Extra Pure benefit laboratory and research activities?
A: Its high purity minimizes impurities that could affect analytical accuracy or experiment results. This makes it ideal for demanding laboratory, analytical, and pharmaceutical research, enhancing data reliability and process efficiency.
Tell us about your requirement

Price:
Quantity
Select Unit
- 50
- 100
- 200
- 250
- 500
- 1000+
Additional detail
Mobile number
Email
More Products in Laboratory Chemicals Category
ACACIA
Price 510 INR
Minimum Order Quantity : 500 Grams
Type : Industrial Lab Chemicals
Grade : AR
Purity(%) : 99%
CAS No : (CAS No.9000015)
AMMONIUM PHOSPHATE monobasic
Price 1195 INR / Gram
Minimum Order Quantity : 500 Grams, ,
Type : University Lab Chemicals
Grade : Laboratory Grade
Purity(%) : 99%
CAS No : 7722761
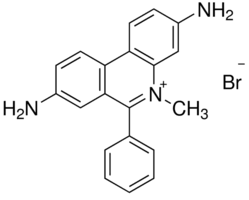
DIMIDIUM BROMIDE
Price 949 INR / Gram
Minimum Order Quantity : 100mg Grams
Type : School Lab Chemicals
Grade : lr ,ar
Purity(%) : 98%
CAS No : 518672
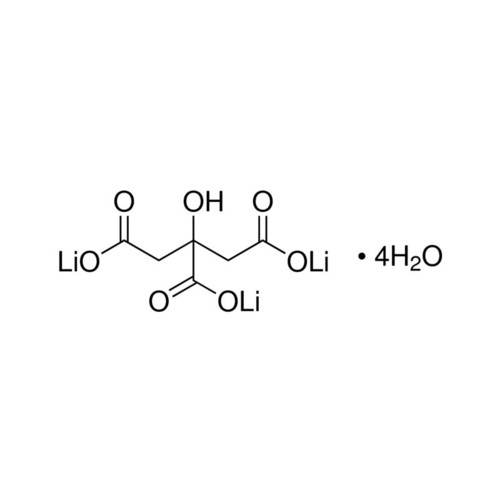
tri-LITHIUM CITRATE AR (tetrahydrate)
Price 300 INR / Kilograms
Minimum Order Quantity : 25gm Kilograms
Type : University Lab Chemicals
Grade : AR
Purity(%) : 99.5%
CAS No : 6080586
We are deals in Lab Chemicals.
"Only deals in retail accepting orders upto 500ml only".
"Only deals in retail accepting orders upto 500ml only".
 |
ALPHA CHEMIKA
All Rights Reserved.(Terms of Use) Developed and Managed by Infocom Network Private Limited. |
 English
English Spanish
Spanish French
French German
German Italian
Italian Chinese (Simplified)
Chinese (Simplified) Japanese
Japanese Korean
Korean Arabic
Arabic Portuguese
Portuguese


 Send Inquiry
Send Inquiry